Abstract
We have implemented a drug screening platform for evaluation of multiple myeloma (MM) relevant therapeutics alone or in combination, providing immediate actionable results. We assembled 80 assay-ready, MM relevant FDA approved drugs and clinical trial therapeutics. This panel was evaluated in 25 MM and 10 Non-Hodgkin lymphoma cell lines, and in 65 primary MM samples using a viability endpoint. The panel includes venetoclax, a selective BCL-2 inhibitor that is currently in clinical development for MM as a single agent or in combination with bortezomib and dexamethasone. Single agent clinical trial results have shown that venetoclax is active in 21% of all relapsed refractory MM patients, 40% of patients with a t(11;14) and only 9% of patients lacking this translocation. It is unclear why not all patients with this translocation respond and who the 9% of non-translocated responders are. We were interested to know if our assay exhibited the same targeted pattern of activity and in assembling a genomic database to interrogate these questions.
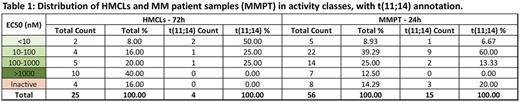
The cellular efficacies of the MM drug panel, including venetoclax, were measured using the CellTiter Glo assay (Promega), in 25 MM cell lines using 7 drug doses covering a broad concentration range at both 24 and 72 hour time points. Concurrent with clinical observations, venetoclax presents a differential activity profile (Table 1). At 72h drug incubation, 75% of t(11;14) MM cell lines responded with an EC50<100nM but only 3 of 21 (14%) of translocation negative cell lines responded (overall response rate of 24%). Ex vivo sensitivity to venetoclax was also measured following 24h drug exposure in CD138+ selected tumor cells from 56 MM patients, 15 of which have a t(11;14). A majority of patient samples (85%) exhibit a dose response curve to venetoclax, but a broad EC50 range is observed (0.59 to 4900 nM). Twenty-seven patient samples (48% of all those tested) were inhibited by venetoclax at <100 nM concentrations, 10 of the 15 patients with t(11;14) responded at <100 nanomolar EC50, accounting for 67% of the samples with that translocation. Two patients with a t(6;14) also responded. For patients who lack either cyclin D1/D3 translocations (n=39), 15 responded with efficacies <100nM, or 38%. These trends are confirmed by median efficacies, with a median EC50 for t(11;14) samples of 29nM, much lower than the 118nM median observed for samples without this translocation. Nevertheless 17 of 41 patients lacking the t(11;14) are sensitive below 100nM EC50, a group which clearly deserves a deeper genomic analysis and clinical correlation.
Using venetoclax as a base drug, we then studied all possible combinations with the 79 other agents of the MM drug panel in sensitive and resistant HMCLs. The cytotoxic response of the base and combined drugs was measured in 5-point 2-fold drug dilutions centered on their respective EC50s. Primary combination screen results were scored using consensus performance metrics from Chou-Talalay, Highest Single Agent (HSA), and Bliss independence models. Combinations predicted synergistic by at least 2 methods were confirmed in a matrix format. The screen combining venetoclax and the drug panel was designed to identify synergistic combinations that enhance venetoclax response in groups that lack t(11;14) and have either intermediate sensitivity (OPM2) or are resistant (AMO1). In OPM2, 25 drugs were found to synergize with venetoclax with a combination index CI < 0.2 (strong synergism), vs 1 non overlapping drug in the resistant HMCL, AMO1. The confirmation of these findings in a matrix format and in sensitive cell lines will be reported.
In summary, our data corroborates the clinical observation of a strong pattern for venetoclax response and t(11;14) through drug screening in primary patient materials ex vivo with a 24 hour read out. Using this strategy we will begin to add drug combinations of high promise to our drug screening platform to develop the map of drug synergism in multiple myeloma.
Acknowledgments: We thank the Multiple Myeloma Research Foundation for funding this project.
Stewart: Bristol-Myers Squibb: Consultancy; Janssen: Consultancy; Amgen: Consultancy; Celgene: Consultancy; Roche: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal